
Page 1 of 6
Coverage Policy Number:
0152
Cigna Medical Coverage Policy
Subject
Reduction Mammoplasty
Effective Date ............................ 8/15/2014
Next Review Date ...................... 8/15/2015
Coverage Policy Number ................. 0152
Table of Contents
Coverage Policy .................................................. 1
General Background ........................................... 2
Coding/Billing Information ................................... 5
References .......................................................... 5
Hyperlink to Related Coverage Policies
Breast Reconstruction following Mastectomy
or Lumpectomy
Gender Reassignment Surgery
Mammography
Prophylactic Mastectomy
Surgical Treatment of Gynecomastia
INSTRUCTIONS FOR USE
The following Coverage Policy applies to health benefit plans administered by Cigna companies. Coverage Policies are intended to provide
guidance in interpreting certain standard Cigna benefit plans. Please note, the terms of a customer’s particular benefit plan document
[Group Service Agreement, Evidence of Coverage, Certificate of Coverage, Summary Plan Description (SPD) or similar plan document] may
differ significantly from the standard benefit plans upon which these Coverage Policies are based. For example, a customer’s benefit plan
document may contain a specific exclusion related to a topic addressed in a Coverage Policy. In the event of a conflict, a customer’s benefit
plan document always supersedes the information in the Coverage Policies. In the absence of a controlling federal or state coverage
mandate, benefits are ultimately determined by the terms of the applicable benefit plan document. Coverage determinations in each specific
instance require consideration of 1) the terms of the applicable benefit plan document in effect on the date of service; 2) any applicable
laws/regulations; 3) any relevant collateral source materials including Coverage Policies and; 4) the specific facts of the particular
situation. Coverage Policies relate exclusively to the administration of health benefit plans. Coverage Policies are not recommendations for
treatment and should never be used as treatment guidelines. In certain markets, delegated vendor guidelines may be used to support
medical necessity and other coverage determinations. Proprietary information of Cigna. Copyright ©2014 Cigna
Coverage Policy
Coverage for reduction mammoplasty is dependent on benefit plan language, may be subject to the
provisions of a cosmetic and/or reconstructive surgery benefit and may be governed by state and/or
federal mandates. Under many benefit plans, reduction mammoplasty is not covered when performed
solely for the purpose of altering appearance or self-esteem or to treat psychological symptomatology
or psychosocial complaints related to one’s appearance. In addition, macromastia surgeries are
specifically excluded under some benefit plans. Please refer to the applicable benefit plan language to
determine the terms and conditions of coverage.
Cigna covers breast reduction surgery on the nondiseased/contralateral breast when performed to
produce a symmetrical appearance following a mastectomy or lumpectomy.
If coverage for reduction mammoplasty is available, the following conditions of coverage apply.
Cigna covers reduction mammoplasty for symptomatic macromastia as medically necessary when ALL
of the following criteria have been met:
• The individual is at least 18 years of age or breast growth is complete.
• Macromastia is causing at least ONE of the following conditions/symptoms with documented failure of at
least one continuous three-month trial of appropriate medical management:
shoulder, upper back/neck pain, and/or ulnar nerve palsy for which no other etiology has been
found on appropriate evaluation

Page 2 of 6
Coverage Policy Number:
0152
intertrigo, dermatitis, eczema, or hidradenitis at the inframammary fold
• The potential causes of the above conditions/symptoms, other than breast size (e.g., intervertebral disc
disorder, arthritis and rheumatologic disorders) have been evaluated and ruled out OR breast size has
been documented as exacerbating the underlying condition (e.g., intervertebral disc disorder, arthritis
and rheumatologic disorders) contributing to symptoms.
• Preoperative photographs confirm the presence of BOTH of the following:
significant breast hypertrophy
shoulder grooving from bra straps and/or intertrigo if stated to be present
• Average weight of tissue planned to be removed in each breast is above the 22nd percentile on the
Schnur Sliding Scale (see Appendix A) based on the individual’s body surface area (BSA).
Cigna does not cover reduction mammoplasty for either of the following indications because it is
considered cosmetic in nature and not medically necessary:
• Surgery is being performed to treat psychological symptomatology or psychosocial complaints, in the
absence of significant physical, objective signs.
• Surgery is being performed for the sole purpose of improving appearance.
Cigna does not cover suction lipectomy or ultrasonically-assisted suction lipectomy (liposuction) as a
sole method of treatment for symptomatic macromastia because such treatment is considered
unproven in the treatment of symptomatic macromastia.
General Background
Macromastia (i.e., female breast hypertrophy) is the development of abnormally large breasts. Normal breast
development begins at approximately five weeks’ gestation and continues until a woman is in her early twenties,
with the rate of development and degree of asymmetry often varying. Spontaneous massive growth of the
breasts during puberty and adolescence is thought to be the result of excessive end-organ sensitivity to gonadal
hormones. It is more commonly bilateral, often occurs over a brief period, and most commonly affects
adolescent girls. Management is individualized and may range from reassurance or the use of supportive
brassieres. It is recommended that surgery be delayed until late adolescence to allow complete breast
development (McGrath and Pomerantz, 2012; DeSilva and Merritt, 2011).
The presence of macromastia may cause clinical manifestations when the excessive breast weight adversely
affects the supporting structures of the shoulders, neck and trunk. Increased weight on the shoulders can cause
pain, fatigue in the cervical and thoracic spine, which can lead to poor posture, thoracic kyphosis and occipital
headaches. Grooving or ulceration of the skin on the shoulders, pressure on the brachial plexus causing
neurological symptoms in the arms and skin conditions occurring at the inframammary fold such as intertrigo,
dermatitis, eczema, or hidradenitis (inflammation of the apocrine sweat glands resulting in obstruction of the
ducts) may also exist. The presence of these persistent signs and painful symptoms distinguish macromastia
from large, normal breasts and may prompt the need for surgical intervention (McGrath and Pomerantz, 2012;
American Society of Plastic Surgeons [ASPS], 2011; Schnur, et al., 1997).
Medical management of conditions/symptoms can include any of the following: weight loss, adequate bra
support (proper fit and wide strap support): nonsteroidal anti-inflammatory drugs (NSAIDS)/analgesia; and
physical therapy, when a functional impairment exists (Collins, et al., 2002).
Reduction mammoplasty is the surgical excision of a substantial portion of the breast, including the skin and the
underlying glandular tissue, until a clinically normal size is obtained. Relocation of the nipple, which may result
in decreased sensation and altered lactation, may also be required during this procedure. Therefore, it has been
recommended that surgery should not be performed on an individual until the breasts are fully developed.
Complications range from mild to severe and may be early or late. The most common early complication
Page 3 of 6
Coverage Policy Number:
0152
independent of reduction technique is delayed wound healing. Late complications can include, but are not
limited to, seroma, scars and pseudoptosis (McGrath and Pomerantz, 2012; Nahai, et al., 2008; Greydanus, et
al., 2006).
The Schnur Sliding Scale is an evaluation tool that may be used to determine the appropriate amount of tissue
to be removed compared to a patient’s total body surface area (BSA). This can be instrumental in determining if
breast reduction is being planned for a purely cosmetic reason or as a medically necessary procedure. In a
survey of plastic surgeons, Schnur et al. (1991) concluded that women whose removed breast weight was less
than the 5th percentile sought the procedure for cosmetic reasons and all women whose breast weight was
greater than the 22nd percentile sought the procedure for medical reasons. A calculation for BSA is: BSA (in m
2
)
= [height (cm)]
0.718
X [weight (kilograms [kg])]
0.427
X .007449.
Breast tissue regrowth following initial breast reduction in adolescence has been reported (Greydanus, et al.,
2006). The growth of the female breast is generally described by five stages referred to as Tanner stages or
sexually maturity rating (SMR) stages. A number of clinical correlations are noted with the SMR stages,
including the timing of breast reduction at stage V (i.e., mature stage) (DeSilva, et al., 2006). In a review of
elective plastic surgical procedures in adolescence, McGrath and Schooler (2004) stated “Breast development
is variable but usually plateaus at 15–16 years of age. Reduction mammoplasty is postponed until breast
maturity is reached. Occasionally, surgery is considered earlier when severe symptoms are encountered; there
is a risk of recurrent hypertrophy, however.” In general, breast maturity should have been reached prior to
considering breast reduction surgery.
Literature Review
Controlled clinical studies assessing the effectiveness of surgical removal of modest amounts of breast tissue in
reducing neck, shoulder, and back pain and related disabilities in women are lacking. Despite the lack of
controlled studies, reduction mammoplasty has become the standard of care for a subset of individuals with
symptomatic macromastia. Evidence suggests that calculating breast reduction in correlation to each patient’s
body weight and height can have an effect on reducing preoperative signs and persistent physical conditions.
(Cunningham, et al., 2005; Blomqvist, et al., 2004; Souto, et al., 2003; Collins, et al., 2002; Ayhan, et al., 2002;
Bruhlmann, et al., 1998).
Chadbourne et al. (2001) conducted a systematic review and meta-analysis of 29 studies of 4173 patients to
determine whether reduction mammoplasty improves measurable outcomes in women with breast hypertrophy.
Experimental and observational studies were included; no randomized controlled trials were found. Outcomes
assessed were postoperative physical signs and symptoms such as shoulder pain, shoulder (bra strap)
grooving, and quality-of-life domains, such as physical and psychological functioning, and were expressed
primarily as risk differences. The mean body mass index of the patients was 27.5 kg/m
2
in the observational
studies and 29.6 kg/m
2
in the experimental studies. The average tissue mass removed per breast was
approximately 1400 grams. The authors concluded that reduction mammoplasty was associated with a
statistically significant improvement in physical signs and symptoms involving shoulder pain, shoulder grooving,
upper/lower back pain, neck pain, intertrigo, breast pain, headache, and pain/numbness in the hands. The
quality-of-life parameter of physical functioning was also statistically significant, while psychological functioning
was not significant. The evidence suggests that women undergoing reduction mammoplasty for breast
hypertrophy have significant postoperative improvement in preoperative signs and symptoms, quality of life, or
both.
Breast Reduction by Liposuction
Suction lipectomy or ultrasonically-assisted suction lipectomy (liposuction) as a sole procedure has been
introduced as an alternative method in reducing breast size. The effectiveness of liposuction, in terms of
removing glandular breast tissue, rather than fatty tissue in the breast, remains to be demonstrated. Evidence
supporting the effects of this approach on patient outcomes has been limited to case series and there are
minimal long-term data comparing this technique to the standard surgical approach (Maskovitz, et al., 2007;
ECRI, 2014; Sadove, et al., 2005).
Professional Societies/Organizations
American Society of Plastic Surgeons (ASPS): The 2011 update to the 2002 ASPS policy statement,
insurance coverage criteria for third-party payors for reduction mammaplasty, recommends that justification for
reduction mammaplasty should be based on the probability of relieving the clinical signs and symptoms of
Page 4 of 6
Coverage Policy Number:
0152
macromastia, not the degree of breast hypertrophy present (cup size or amount of tissue removed).
Symptomatic breast hypertrophy is defined as a syndrome of persistent neck and shoulder pain, painful
shoulder grooving from brassiere straps, chronic intertriginous rash of the inframammary fold, and frequent
episodes of headache, backache, and neuropathies caused by heavy breasts caused by an increase in the
volume and weight of breast tissue beyond normal proportions. These policy recommendations are based on
the 2011 ASPS evidence based companion guideline for Reduction Mammaplasty.
Use Outside of the US
No relevant information.
Summary
The evidence in the peer-reviewed published literature supports use of reduction mammoplasty to improve signs
and symptoms associated with macromastia. There is a lack of well-designed controlled clinical trials to assess
the amount of breast tissue removed and the reduction of signs and symptoms of macromastia. A patient’s
signs and symptoms and the amount of breast tissue to be resected needs to be evaluated before the
performance of reduction mammoplasty for macromastia.
The effectiveness of suction lipectomy or ultrasonically-assisted suction lipectomy (liposuction) as a sole method
of treatment for symptomatic macromastia in terms of removing glandular breast tissue, rather than fatty tissue
in the breast, remains to be demonstrated. Evidence in the peer-reviewed published literature supporting the
effects of this approach on patient outcomes has been limited to case series and there are minimal long-term
data comparing this technique to the standard surgical approach for reduction mammoplasty.
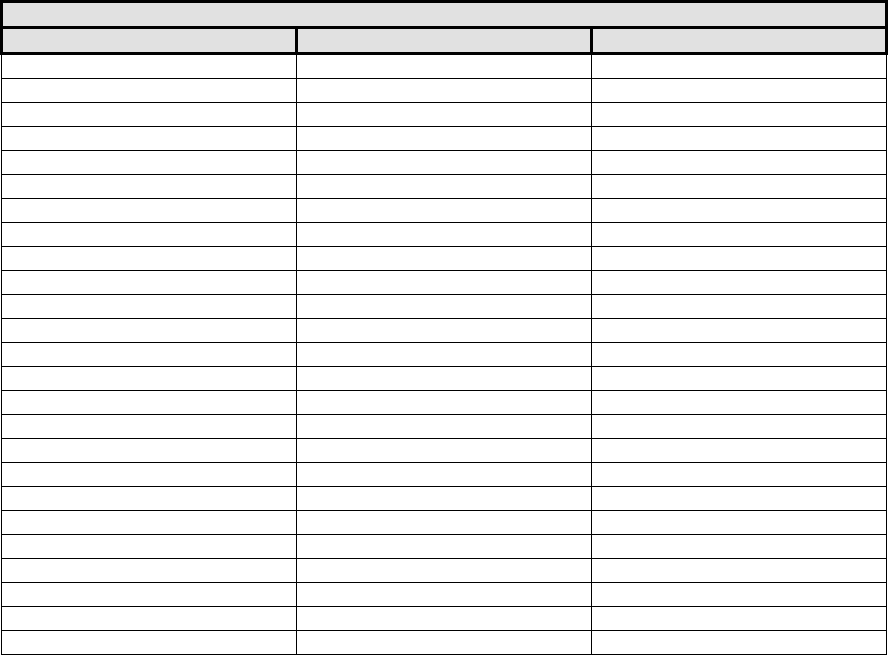
Appendix A
Schnur Sliding Scale
Body Surface Area and Cutoff Weight of Breast Tissue Removed
Breast Reduction (gm)
Body Surface Area (m
2
)
Lower 5%
Lower 22%
1.35
127
199
1.40
139
218
1.45
152
238
1.50
166
260
1.55
181
284
1.60
198
310
1.65
216
338
1.70
236
370
1.75
258
404
1.80
282
441
1.85
308
482
1.90
336
527
1.95
367
575
2.00
401
628
2.05
439
687
2.10
479
750
2.15
523
819
2.20
572
895
2.25
625
978
2.30
682
1068
2.35
745
1167
2.40
814
1275
2.45
890
1393
2.50
972
1522
2.55
1062
1662
Page 5 of 6
Coverage Policy Number:
0152
Schnur Sliding Scale (Schnur, et al., 1991)
Coding/Billing Information
Note: 1) This list of codes may not be all-inclusive.
2) Deleted codes and codes which are not effective at the time the service is rendered may not be eligible
for reimbursement.
Covered as medically necessary:
CPT
®
*
Codes
Description
19318
Reduction mammoplasty
Unproven/Not Covered when performed as a sole method of treatment for symptomatic
macromastia:
CPT* Codes
Description
15877
Suction assisted lipectomy; trunk
*Current Procedural Terminology (CPT
®
)
©
2013 American Medical Association: Chicago, IL.
References
1. American Society of Plastic Surgeons. Reduction Mammaplasty Recommended Criteria for Third-Party
Payer Coverage from the American Society of Plastic Surgeons (ASPS). May 2011. Accessed July 2,
2014. Available at URL address: http://www.plasticsurgery.org
2. American Society of Plastic Surgeons. Reduction Mammaplasty. Evidence-Based Practice Guidelines.
May 2011. Accessed July 2, 2014. Available at URL address:
http://www.guideline.gov/content.aspx?id=34042
3. Ayhan S, Basterzi Y, Yavuzer R, Latifoglu O, Cenetoglu S, Atabay K, Celebi MC. Histologic profiles of
breast reduction specimens. Anesthetic Plast Surg. 2002 May;26(3):203-5.
4. Blomqvist L, Brandberg Y. Three-year follow-up on clinical symptoms and health-related quality of life
after reduction mammaplasty Plast Reconstr Surg. 2004 Jul;114(1):49-54.
5. Bruhlmann Y, Tschopp H. Breast reduction improves symptoms of macromastia and has a long-lasting
effect. Ann Plast Surg. 1998 Sep;41(3):240-5.
6. Chadbourne EB, Zhang S, Gordon MJ, Ro EY, Ross SD, Schnur PL, Schneider-Redden PR. Clinical
outcomes in reduction mammoplasty: a systematic review and meta-analysis of published studies. Mayo
Clin Proc. 2001 May;76(5):503-10.
7. Chao JD, Memmel HC, Redding JF, Egan L, Odom LC, Casas LA. Reduction mammaplasty is a
functional operation, improving quality of life in symptomatic women: a prospective, single-center breast
reduction outcome study. Plast Reconstr Surg. 2002 Dec;110(7):1644-52.
8. Collins ED, Kerrigan CL, Kim M. The effectiveness of surgical and nonsurgical interventions in relieving
the symptoms of macromastia. Plast Reconstr Surg. 2002 Jul;109:1556-66.
9. Cunningham BL, Gear AJ, Kerrigan CL, Collins ED. Analysis of breast reduction complications derived
from the BRAVO study. Plast Reconstr Surg. 2005 May;115(6):1597-604.

Page 6 of 6
Coverage Policy Number:
0152
10. DeSilva NK, Brandt ML. Disorders of the breast in children and adolescents, Part 1: Disorders of growth
and infections of the breast. J Pediatr Adolesc Gynecol. 2006 Oct;19(5):345-9.
11. DeSilva NK, Merritt DF. Breast Concerns. In: Kliegman RM, Stanton BF, St Geme JW, Schor NF,
Behrman RE, editors. Kliegman: Nelson Textbook of Pediatrics. 19
th
ed. Philadelphia, PA: Elsevier;
2011. Ch 545.
12. ECRI Institute. Hotline Response [database online]. Plymouth Meeting (PA): ECRI Institute; 2014 May
13. Liposuction only-breast reduction surgery. Available at URL address: http://www.ecri.org
13. Gonzalez MA, Glickman LT, Aladegbami B, Simpson RL. Quality of life after breast reduction surgery: a
10-year retrospective analysis using the Breast Q questionnaire: does breast size matter? Ann Plast
Surg. 2012 Oct;69(4):361-3.
14. Greydanus DE, Matytsina L, Gains M. Breast Disorders in Children and Adolescents. Prim Care. 2006
Jun;33(2):455-502.
15. Kalliainen LK; ASPS Health Policy Committee. ASPS clinical practice guideline summary on reduction
mammaplasty. Plast Reconstr Surg. 2012 Oct;130(4):785-9.
16. Kocak E, Carruthers KH, McMahan JD. A reliable method for the preoperative estimation of tissue to be
removed during reduction mammaplasty. Plast Reconstr Surg. 2011 Mar;127(3):1059-64.
17. McGrath MH, Pomerantz J. Plastic Surgery. Reduction Mammoplasty. In: Townsend CM, Beuchamp
RD, Evers BM, editors. Townsend: Sabiston Textbook of Surgery, 19
th
ed. Philadelphia, PA: WB
Saunders Company. 2012. pg 1932-33. Ch 69.
18. McGrath MH, Schooler WG. Elective plastic surgical procedures in adolescence. Adolesc Med Clin.
2004 Oct;15(3):487-502.
19. Moskovitz MJ, Baxt SA, Jain AK, Hausman RE. Liposuction breast reduction: a prospective trial in
African American women. Plast Reconstr Surg. 2007 Feb;119(2):718-26; discussion 727-8.
20. Nahai FR, Nahai F. MOC-PSSM CME article: Breast reduction. Plast Reconstr Surg. 2008 Jan;121(1
Suppl):1-13.
21. Sadove R. New observations in liposuction-only breast reduction. Aesthetic Plast Surg. 2005 Jan-
Feb;29(1):28-31. Epub 2005 Mar 17.
22. Schnur PL, Hoehn JG, Ilstrup DM, Cahoy MJ, Chu CP. Reduction mammaplasty: cosmetic or
reconstructive procedure? Ann Plast Surg. 1991 Sep;27(3):232-7.
23. Schnur PL, Schnur DP, Petty PM, Hanson TJ, Weaver Al. Reduction mammoplasty: an outcome study.
Plast Reconstr Surg. 1997 Sep;100(4):875-83.
24. Singh KA, Losken A. Additional benefits of reduction mammaplasty: a systematic review of the
literature. Plast Reconstr Surg. 2012 Mar;129(3):562-70.
25. Souto GC, Giugliani ER, Giugliani C, Schneider MA. The impact of breast reduction surgery on
breastfeeding performance. J Hum Lact. 2003 Feb;19(1):43-9;quiz 66-9, 120.
The registered marks "Cigna" and the "Tree of Life" logo are owned by Cigna Intellectual Property, Inc., licensed for use by Cigna Corporation
and its operating subsidiaries. All products and services are provided by or through such operating subsidiaries and not by Cigna Corporation.
Such operating subsidiaries include Connecticut General Life Insurance Company, Cigna Health and Life Insurance Company, Cigna Behavioral
Health, Inc., Cigna Health Management, Inc., and HMO or service company subsidiaries of Cigna Health Corporation.
